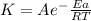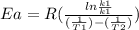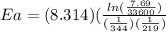Areaction is followed and found to have a rate constant of 3.36 × 104 m-1s-1 at 344 k and a rate constant of 7.69 m-1s-1 at 219 k. determine the activation energy for this reaction. a reaction is followed and found to have a rate constant of 3.36 × 104 m-1s-1 at 344 k and a rate constant of 7.69 m-1s-1 at 219 k. determine the activation energy for this reaction. 12.5 kj/mol 11.5 kj/mol 23.8 kj/mol 58.2 kj/mol 42.0 kj/mol

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 07:30
Compare and contrast the bohr model and the electron cloud models of the atom.
Answers: 1

Chemistry, 22.06.2019 21:30
How can the periodic table be used to predict the behavior of elements?
Answers: 1

Chemistry, 22.06.2019 22:00
Scientists often have to deal with numbers that are either very large or very small. for example, the radius of the sun is approximately 696,000 kilometers, while bacterial cells are as small as 1.9 × 10-4 millimeters. express each number in an alternate form.
Answers: 1

Chemistry, 22.06.2019 23:30
The density of benzene at 15 °c is 0.8787 g/ml. calculate the mass of 0.1500 l of benzene at this temperature. enter your answer in terms of grams
Answers: 2
You know the right answer?
Areaction is followed and found to have a rate constant of 3.36 × 104 m-1s-1 at 344 k and a rate con...
Questions

Computers and Technology, 30.08.2019 20:10




Computers and Technology, 30.08.2019 20:20

History, 30.08.2019 20:20

English, 30.08.2019 20:20

Physics, 30.08.2019 20:20


History, 30.08.2019 20:20

Mathematics, 30.08.2019 20:20


Mathematics, 30.08.2019 20:20


Chemistry, 30.08.2019 20:20

Chemistry, 30.08.2019 20:20


Computers and Technology, 30.08.2019 20:20

Mathematics, 30.08.2019 20:20

Social Studies, 30.08.2019 20:20