
Chemistry, 07.11.2019 18:31 monkeys450
Acertain first-order reaction (a→products) has a rate constant of 9.60×10−3 s−1 at 45 ∘c. how many minutes does it take for the concentration of the reactant, [a], to drop to 6.25% of the original concentration?

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 02:10
Determine the percent sulfuric acid by mass of a 1.61 m aqueous solution of h2so4. %
Answers: 2


Chemistry, 23.06.2019 03:30
Calculate the ph of a .10m nh4cl solution. the kb value for nh3 is 1.8×10^-5
Answers: 1
You know the right answer?
Acertain first-order reaction (a→products) has a rate constant of 9.60×10−3 s−1 at 45 ∘c. how many m...
Questions

Biology, 09.07.2019 23:00




Mathematics, 09.07.2019 23:00

Spanish, 09.07.2019 23:00

History, 09.07.2019 23:00

History, 09.07.2019 23:00

History, 09.07.2019 23:00


History, 09.07.2019 23:00

English, 09.07.2019 23:00


Chemistry, 09.07.2019 23:00

Mathematics, 09.07.2019 23:00



Mathematics, 09.07.2019 23:00

Physics, 09.07.2019 23:00

![\log [A]=\log [A_o]-\frac{kt}{2.303}](/tpl/images/0364/0253/6b0a0.png)
![[A_o]](/tpl/images/0364/0253/dc622.png) = Initial concentration of reactant
= Initial concentration of reactant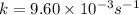
![[A_o]=x](/tpl/images/0364/0253/aecde.png)
![[A]=6.25% of x =0.0625x](/tpl/images/0364/0253/3dc32.png)
![\log [0.0625 x]=\log[x]-\frac{9.60\times 10^{-3} s^{-1}\tmes t}{2.303}](/tpl/images/0364/0253/dd087.png)


