
Chemistry, 05.02.2020 04:54 rileyallen4186pd5tgy
Consider the balanced chemical reaction below. when the reaction was carried out, the calculated theoretical yield for carbon dioxide was 93.7 grams, but the measured yield was 88.3 grams. what is the percent yield?
fe2o3 + 3co --> 2fe + 3co2

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 10:30
Astudent reacts 13 moles of iron with 21 moles of oxygen according to the following equation:
Answers: 1

Chemistry, 22.06.2019 21:00
How many neutrons does an element have if its atomic number is 50 and its mass number is 166
Answers: 1

Chemistry, 22.06.2019 21:50
If e is the symbol for an element, which two of the following symbols represent isotopes of the same element? 1. e2. e3. ea.1 and 2c.1 and 4b.3 and 4d.2 and 3
Answers: 2

Chemistry, 22.06.2019 22:00
Choose all the answers that apply. fluorine (f) has an atomic number of 9 and an atomic weight of 18.99. fluorine has a. 9 protons b. 10 neutrons c. 18 electrons d. an atomic mass of 19 e. at least one isotope
Answers: 1
You know the right answer?
Consider the balanced chemical reaction below. when the reaction was carried out, the calculated the...
Questions

Business, 09.01.2020 02:31

Chemistry, 09.01.2020 02:31




Mathematics, 09.01.2020 02:31



Mathematics, 09.01.2020 02:31


Mathematics, 09.01.2020 02:31

Mathematics, 09.01.2020 02:31

Mathematics, 09.01.2020 02:31

English, 09.01.2020 02:31


English, 09.01.2020 02:31



Chemistry, 09.01.2020 02:31

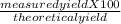
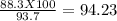 %
%

