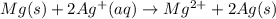An electrochemical cell has the following standard cell notation.
mg(s) | mg^2+ (aq) ||...

An electrochemical cell has the following standard cell notation.
mg(s) | mg^2+ (aq) || aq^+(aq) | aq(s)
write a blanked redox equation for the cell using the oxidation and reduction half reactions. (be sure to equalize charge by multiplying by the correct numbers before adding and simplifying)

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 17:30
When the earth was formed and cooled, why did nickel and iron end up in the center of the earth while basalt and granite ended up in the outer layers
Answers: 3

Chemistry, 22.06.2019 09:20
Give the orbital configuration of the phosphorus (p) atom.
Answers: 1

Chemistry, 22.06.2019 14:00
What mass of natural gas (ch4) must you burn to emit 276 kj of heat?
Answers: 1

Chemistry, 22.06.2019 18:00
The human activities in two locations are described below: location a: rampant use of plastic containers location b: excessive use of pesticides and fertilizers which statement is most likely true? location a will have poor air quality because plastic is biodegradable. location a will experience water scarcity because plastic absorbs moisture. the population of honeybees will increase in location b because production of crops will increase. the population of fish in location b will decrease because the water is contaminated.
Answers: 1
You know the right answer?
Questions




Physics, 29.01.2020 23:04

Mathematics, 29.01.2020 23:04

Mathematics, 29.01.2020 23:04








Mathematics, 29.01.2020 23:05


Chemistry, 29.01.2020 23:05

Mathematics, 29.01.2020 23:05

Mathematics, 29.01.2020 23:05

Mathematics, 29.01.2020 23:05

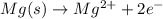 ....(1)
....(1)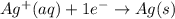 ...(2)
...(2)