
Chemistry, 04.02.2020 16:58 genesisdiaz1352
Calculate the number of atoms of carbon (c) in 340. cm3 of the colorless gas methylacetylene at 0 °c and atmospheric pressure, where its density is 1.79×10-3 g cm-3. the molecular formula of methylacetylene is c3h4.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 17:30
How many grams of magnesium metal will react completely with 8.3 liters of 5.5 m hcl? show all of the work needed to solve this problem. mg (s) + 2hcl (aq) → mgcl2 (aq) + h2 (g)
Answers: 3

Chemistry, 21.06.2019 21:30
Sex cells from female reproductive organ? 1) mitosis 2) fertilization 3) zygote 4) eggs 5) meiosis 6) sperm
Answers: 2

Chemistry, 22.06.2019 01:00
According to the tide table below what time of day will the highest tide occur?
Answers: 1

Chemistry, 22.06.2019 06:00
If you burn 10 kilograms of wood in a fire (combustion) what is the weight of the products after the fire has finished burning the wood?
Answers: 3
You know the right answer?
Calculate the number of atoms of carbon (c) in 340. cm3 of the colorless gas methylacetylene at 0 °c...
Questions

Chemistry, 01.12.2021 14:00

Mathematics, 01.12.2021 14:00

Mathematics, 01.12.2021 14:00



History, 01.12.2021 14:00

Physics, 01.12.2021 14:00

Mathematics, 01.12.2021 14:00




Mathematics, 01.12.2021 14:00

Mathematics, 01.12.2021 14:00


English, 01.12.2021 14:00

Mathematics, 01.12.2021 14:00

Chemistry, 01.12.2021 14:00



Mathematics, 01.12.2021 14:00

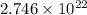
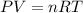
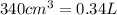 (Conversion Factor:
(Conversion Factor: 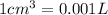 )
)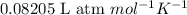
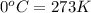 (Conversion factor:
(Conversion factor: 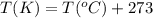 )
)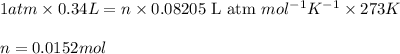
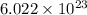 number of atoms
number of atoms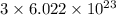 number of C-atoms.
number of C-atoms.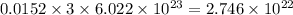 number of C-atoms.
number of C-atoms.

