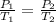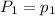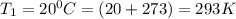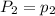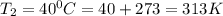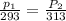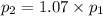Chemistry, 05.02.2020 02:52 alexandroperez13
To understand the ideal gas law and be able to apply it to a wide variety of situations. the absolute temperature t, volume v, and pressure p of a gas sample are related by the ideal gas law, which states that pv=nrt. here n is the number of moles in the gas sample and r is a gas constant that applies to all gases. this empirical law describes gases well only if they are sufficiently dilute and at a sufficiently high temperature that they are not on the verge of condensing. in applying the ideal gas law, p must be the absolute pressure, measured with respect to vacuum and not with respect to atmospheric pressure, and t must be the absolute temperature, measured in kelvins (that is, with respect to absolute zero, defined throughout this tutorial as ^ -273˚c). if p is in pascals and v is in cubic meters, use r=8.3145j/(mol x k). if p is in atmospheres and v is in liters, use r=0.08206l x atm/(mol x k) instead. part a a gas sample enclosed in a rigid metal container at room temperature (20.0˚c) has an absolute pressure p1. the container is immersed in hot water until it warms to 40.0˚c. what is the new absolute pressure p2?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:50
What are transitions between a liquid and a solid called? identify which way they are transitioning
Answers: 2


Chemistry, 22.06.2019 20:00
What is the molarity of the solution produced when 145 g of nacl is dissolved in sufficient water to prepare 2.75 l of solution?
Answers: 1

Chemistry, 23.06.2019 10:30
Ethyl alcohol, also known as ethanol, has a density of 0.79 g/ml. what is the volume, in quarts, of 1.95 kg of this alcohol?
Answers: 2
You know the right answer?
To understand the ideal gas law and be able to apply it to a wide variety of situations. the absolut...
Questions


History, 06.11.2020 05:50

English, 06.11.2020 05:50

Mathematics, 06.11.2020 05:50

Chemistry, 06.11.2020 05:50

Health, 06.11.2020 05:50




Mathematics, 06.11.2020 05:50

Advanced Placement (AP), 06.11.2020 05:50


Chemistry, 06.11.2020 05:50

History, 06.11.2020 05:50

Mathematics, 06.11.2020 05:50

Biology, 06.11.2020 05:50

Mathematics, 06.11.2020 05:50



Mathematics, 06.11.2020 05:50

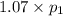
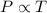 (At constant volume and number of moles)
(At constant volume and number of moles)