
Chemistry, 03.12.2019 02:31 carlydays3331
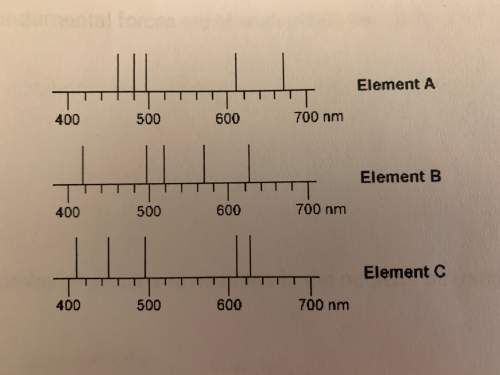
Ahigh school student acquired an emission spectrum of an unknown sample. he knew the unknown sample contained one of the elements, whose spectra are show below. the emission spectrum of his sample showed a strong emission at 610 nm but not at 480 nm.
a. which element did his unknown sample contain?
b. element a above has a strong emission around 670nm. does this emission line represent a lower energy or higher energy transition than the emission line at 428nm?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 17:00
Which property of a rock remains unchanged by mechanical weathering? a. total surface area b. size and shape c. mineral composition d. sharpness
Answers: 1

Chemistry, 22.06.2019 23:30
The sum of the oxidation numbers in a neutral compound is always
Answers: 2

Chemistry, 23.06.2019 05:00
Which of the following describes qualitative data? a) recording the temperature of a solid as it is warmed. b) noting the color of a solution as it is heated. c) measuring the volume of an object by water displacement. d) taking the mass of an object using a balance.
Answers: 2

Chemistry, 23.06.2019 09:30
The mass of a proton is approximately equal to the mass of
Answers: 1
You know the right answer?
Ahigh school student acquired an emission spectrum of an unknown sample. he knew the unknown sample...
Questions



Geography, 02.12.2020 19:00


Biology, 02.12.2020 19:00

Mathematics, 02.12.2020 19:00

Mathematics, 02.12.2020 19:00

History, 02.12.2020 19:00


Mathematics, 02.12.2020 19:00

Mathematics, 02.12.2020 19:00

Mathematics, 02.12.2020 19:00

Mathematics, 02.12.2020 19:00


Spanish, 02.12.2020 19:00



Mathematics, 02.12.2020 19:00

Geography, 02.12.2020 19:00

Mathematics, 02.12.2020 19:00



