
Chemistry, 17.10.2019 01:00 HighSchool97654
How many moles of water are produced when 3.0 moles of hydrogen gas react with 1.8 moles of oxygen gas?
a. 3.0 moles of water
b. 3.6 moles of water
c. 5.3 moles of water
d. 7.0 moles of water

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 11:30
Voltaic cells produce a positive overall charge. what does this indicate? a. the reaction is likely to be endothermic. b. the reaction is spontaneous. c. the reaction is not likely to occur. d. the reaction is not spontaneous.
Answers: 3

Chemistry, 22.06.2019 12:00
What term is applied to a scientist who studies ancient life, including animal and plant fossils a. anthropologist b. dendroclimatologist c. geophysicist d. paleontologist
Answers: 2

Chemistry, 22.06.2019 16:30
For the reaction shown, calculate how many moles of no2 form when each of the following completely reacts. 2n2o5(g)→4no2(g)+o2(g) part a 1.0 mol n2o5 express your answer using two significant figures. nothing mol m o l request answer part b 5.4 mol n2o5 express your answer using two significant figures.
Answers: 2

Chemistry, 22.06.2019 23:10
Afusion reaction takes place between carbon and another element. neutrons are released, and a different element is formed. the different element is a) lighter than helium.b)heavier than helium.c)the same weight as helium.d)dependent on the element that reacted with carbon.
Answers: 3
You know the right answer?
How many moles of water are produced when 3.0 moles of hydrogen gas react with 1.8 moles of oxygen g...
Questions




Chemistry, 20.11.2020 23:10



Mathematics, 20.11.2020 23:10


History, 20.11.2020 23:10


English, 20.11.2020 23:10

Mathematics, 20.11.2020 23:10


Mathematics, 20.11.2020 23:10

Biology, 20.11.2020 23:10


Advanced Placement (AP), 20.11.2020 23:10


Mathematics, 20.11.2020 23:10

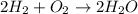
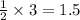 moles of oxygen.
moles of oxygen.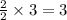 moles of water.
moles of water.


