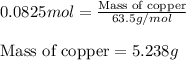Chemistry, 08.10.2019 23:30 weirdoal567
Achemist wants to extract copper metal from copper chloride solution. the chemist places 1.50 grams of aluminum foil in a solution of 14 grams of copper (ii) chloride. (single replacement reaction.) what best explains the state of the reaction mixture afterward?
a. less than 6.0 grams of copper (ii) is formed, and some aluminum is left in the reaction mixture.
b. more than 6.5 grams of copper (ii) is formed, and some aluminum is left in the reaction mixture.
c. less than 6.0 grams of copper (ii) is formed, and some copper chloride is left in the reaction mixture.
d. more than 6.5 grams of copper (ii) is formed, and some copper chloride is left in the reaction mixture.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 16:10
Agas mixture with a total pressure of 745 mmhg contains each of the following gases at the indicated partial pressures: co2, 245 mmhg ; ar, 119 mmhg ; and o2, 163 mmhg . the mixture also contains helium gas. part a what is the partial pressure of the helium gas? phe p h e = nothing mmhg request answer part b what mass of helium gas is present in a 10.2-l sample of this mixture at 283 k ? m m = nothing g request answer
Answers: 1

Chemistry, 21.06.2019 17:30
This is a mixture that has the same composition throughout.
Answers: 1

Chemistry, 21.06.2019 21:00
Mrs. smith ordered a root beer float (vanilla ice cream + root beer). mrs. smith noticed that the three states of matter (solid, liquid, and gas) all existed simultaneously in her root beer float. a. identify each phase of matter in the root beer float. b. describe the particles of all three phases of matter in the root beer float. (how are the particles arranged and moving? ) c. identify one phase change you would see in a root beer float and described what causes this change.
Answers: 2

Chemistry, 21.06.2019 21:30
Which statements are true about electrolysis? check all that apply. electrolysis requires an acid be present. electrolysis is described by two half-reactions. electrolysis is not an industrial process. electrolysis results in commercially valuable products. electrolysis involves the transfer of electrons. reduction results in the loss of electrons. oxidation results in the loss of electrons.
Answers: 1
You know the right answer?
Achemist wants to extract copper metal from copper chloride solution. the chemist places 1.50 grams...
Questions

Mathematics, 19.11.2020 16:20


Mathematics, 19.11.2020 16:20

Mathematics, 19.11.2020 16:20

Social Studies, 19.11.2020 16:20

English, 19.11.2020 16:20

Physics, 19.11.2020 16:20

English, 19.11.2020 16:20

English, 19.11.2020 16:20

Mathematics, 19.11.2020 16:20

French, 19.11.2020 16:20


Mathematics, 19.11.2020 16:20

World Languages, 19.11.2020 16:20


Biology, 19.11.2020 16:20


Mathematics, 19.11.2020 16:20

Mathematics, 19.11.2020 16:20

Mathematics, 19.11.2020 16:20

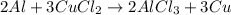
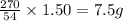 of copper chloride.
of copper chloride.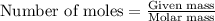 ....(1)
....(1)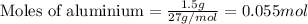
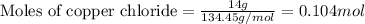
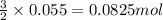 of copper chloride.
of copper chloride.