Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 02:30
If a 12-v battery is connected to a circuit that has a current of 3.0 a, what is the total resistance in the circuit? 36 ohms 4 ohms 0.25 ohms
Answers: 1

Chemistry, 22.06.2019 05:00
Cucl2 + 2nano3 cu(no3)2 + 2nacl what is the percent yield of nacl if 31.0 g of cucl2 reacts with excess nano3 to produce 21.2 g of nacl? 49.7% 58.4% 63.6% 78.7%
Answers: 1

Chemistry, 22.06.2019 05:30
What type of reaction is shown below? check all that apply. 2h2o2 → 2h2o + o2 synthesis decomposition combustion
Answers: 3

You know the right answer?
C3h8 + 5o2 = 3co2 + 4h2o when 44.0 grams of propane (c3h8) under goes complete combustion, how many...
Questions



Computers and Technology, 10.03.2020 20:36



Mathematics, 10.03.2020 20:36



Chemistry, 10.03.2020 20:36




Mathematics, 10.03.2020 20:36

Chemistry, 10.03.2020 20:36






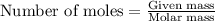 ....(1)
....(1)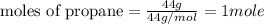
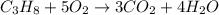
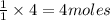 of water.
of water.