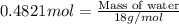Chemistry, 18.01.2020 06:31 webbjalia04
In the following balanced equation 2c2h6 + 7o2 --> 4co2 + 6h20 if 7.0 g of c2h6 react with 18g of o2, how many grams of water will be produced?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 02:30
When svante arrhenius first proposed his acid-base theory, he was a doctoral candidate. his professors thought his ideas were unfounded. within a decade, the arrhenius theory of acid-base was widely accepted and praised within the scientific world. arrhenius defined acids as compounds having ionizable hydrogen and bases as compounds with ionizable a) barium. b) hydronium. c) hydroxide. d) oxygen.
Answers: 3

Chemistry, 22.06.2019 05:50
According to coulomb's law, how would the electrical force between particles change if the product of their electrical charge increased?
Answers: 1

Chemistry, 22.06.2019 14:00
The content of manganese (mn) in steel was determined spectrophotometrically and with the use of the standard addition method. an unknown sample of mn from a digested steel sample gave an absorbance of 0.185 when analyzed spectrophotometrically. when 5.00 ml of solution containing 95.5 ppm mn was added to 50.0 ml of the unknown steel solution (digested sample), the absorbance was 0.248. calculate the concentration, in parts-per-million (ppm), of mn in the digested steel sample solution.
Answers: 3

You know the right answer?
In the following balanced equation 2c2h6 + 7o2 --> 4co2 + 6h20 if 7.0 g of c2h6 react with 18g o...
Questions

Health, 27.03.2020 11:32

Mathematics, 27.03.2020 11:33

Mathematics, 27.03.2020 11:33




Mathematics, 27.03.2020 11:43



Chemistry, 27.03.2020 11:43

Biology, 27.03.2020 11:44

Social Studies, 27.03.2020 11:45


History, 27.03.2020 11:45




Mathematics, 27.03.2020 11:47


Biology, 27.03.2020 11:47

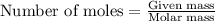 ...(1)
...(1)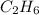 :
: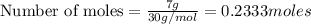
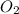 :
: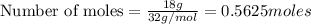
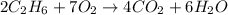
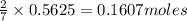 of ethane.
of ethane.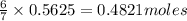 of water.
of water.