Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:50
Using a value of ksp = 1.8 x 10-2 for the reaction pbcl2 pb+2(aq) + 2cl -(aq). if the value of ksp was determined to be only 1.2 x 10-2: too much solid has dissolved. additional precipitate is forming. the solution is unsaturated. the ions are now combining to reduce their concentrations.
Answers: 3

Chemistry, 22.06.2019 11:00
The diagram below shows the different phase transitions that occur in matter. which arrow represents the transition in which dew is formed?
Answers: 1

Chemistry, 22.06.2019 14:20
Which of the following are sources of revenue for media companies? a. direct sales to producers b.advertising and subscriptions c. online purchase d. capital investments
Answers: 1

Chemistry, 22.06.2019 20:10
Suppose you mix one mole of sulfuric acid (h2so4) with 1 mole of sodium hydroxide(naoh). why does the ph of the solution remain below 7? ( explain so i can get better understanding! )
Answers: 2
You know the right answer?
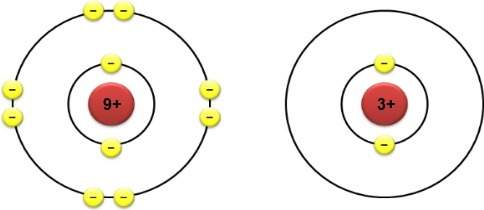
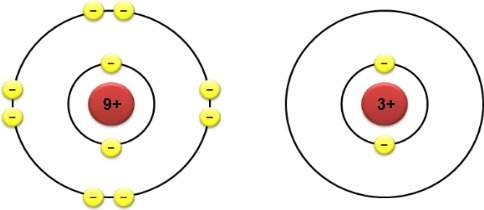
The electron configurations of two different atoms are shown below. each yellow electron has a charg...
Questions

Mathematics, 29.09.2019 04:30

Mathematics, 29.09.2019 04:30

English, 29.09.2019 04:30





English, 29.09.2019 04:30

Mathematics, 29.09.2019 04:30


Biology, 29.09.2019 04:30

Mathematics, 29.09.2019 04:30

English, 29.09.2019 04:30

Mathematics, 29.09.2019 04:30


Social Studies, 29.09.2019 04:30

History, 29.09.2019 04:30



History, 29.09.2019 04:30