Read the given expression. x = number of protons − number of core electrons which of the following explains the identity of x and its trends across a period? x is the effective nuclear charge, and it remains constant across a period. x is the screening constant, and it remains constant across a period. x is the effective nuclear charge, and it increases across a period. x is the screening constant, and it increases across a period.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 07:30
11. phosphorus-32 is radioactive and has a half life of 14 days. how much of a 124 mg sample of phosphorus-32 is present after 56 days? a) 7.75 mg b) 15.5 mg c) 31.0 mg d) 62.0 mg
Answers: 3

Chemistry, 22.06.2019 19:10
How does the atmosphere to make earth livable? check all that apply. causes the seasons contains oxygen provides warmth creates important nutrients blocks harmful energy from the sun plz like !
Answers: 2


Chemistry, 22.06.2019 22:30
Vi limitens. vastery test select the correct answer. which statement explains why large atoms are more reactive than small atoms? a. large atoms have valence electrons farther from the nucleus and lose them more readily. b. large atoms have greater ionization energy, which they can utilize during a reaction. c. large atoms have a greater number of electrons that they can lose during a reaction. d. large atoms have more energy levels, so they have more energy to pass on in a reaction. reset next
Answers: 3
You know the right answer?
Read the given expression. x = number of protons − number of core electrons which of the following e...
Questions


Social Studies, 01.08.2019 16:00



English, 01.08.2019 16:00

Mathematics, 01.08.2019 16:00

Social Studies, 01.08.2019 16:00


Social Studies, 01.08.2019 16:00

Computers and Technology, 01.08.2019 16:00



Social Studies, 01.08.2019 16:00



Chemistry, 01.08.2019 16:00

History, 01.08.2019 16:00


History, 01.08.2019 16:00

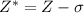
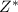 = effective nuclear charge
= effective nuclear charge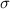 = Screening constant
= Screening constant