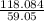Chemistry, 21.06.2019 15:30 hannah5143
Determine the empirical formula of a compound containing 40.6 grams of carbon, 5.1 grams of hydrogen, and 54.2 grams of oxygen. in an experiment, the molar mass of the compound was determined to be 118.084 g/mol. what is the molecular formula of the compound? for both questions, show your work or explain how you determined the formulas by giving specific values used in calculations.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 06:30
If 1.8 l of water is added to 2.5l of a 7.0 m koh solution, what is the molarity of the new solution
Answers: 1

Chemistry, 23.06.2019 00:30
Quickly what are the following of organisms that existed over a wide area but only for a limited time period called a.soft fossils b.mold fossils c.index fossils d.trace fossils
Answers: 1

Chemistry, 23.06.2019 00:30
Arrange the elements in order of increasing electronegativity. use the periodic table to you arrange the elements. p o k mg
Answers: 2

You know the right answer?
Determine the empirical formula of a compound containing 40.6 grams of carbon, 5.1 grams of hydrogen...
Questions



History, 30.10.2020 20:50


Mathematics, 30.10.2020 20:50

History, 30.10.2020 20:50






English, 30.10.2020 20:50


Mathematics, 30.10.2020 20:50

Mathematics, 30.10.2020 20:50


Mathematics, 30.10.2020 20:50


Social Studies, 30.10.2020 20:50

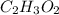 and molecular formula is
and molecular formula is 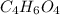 .
. 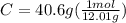

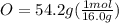
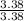 = 1
= 1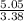 = 1.5
= 1.5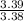 = 1
= 1