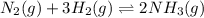Chemistry, 23.06.2019 03:00 draveon6925
Achemical equilibrium between gaseous reactants and products is shown. n2(g) + 3h2(g) ⇌ 2nh3(g) how will the reaction be affected if the pressure on the system is increased? it will shift toward the reactant side as there is lower pressure on the reactant side. it will shift toward the product side as there is higher pressure on the product side. it will shift toward the reactant side as there are a greater number of moles of gas on the reactant side. it will shift toward the product side as there are a fewer number of moles of gas on the product side.

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 19:40
What type of electromagnetic waves does the human eye see as the colors red blue or green a visible light waves b radio waves c infrared waves d microwaves
Answers: 1

Chemistry, 22.06.2019 20:00
Many free radicals combine to form molecules that do not contain any unpaired electrons. the driving force for the radical–radical combination reaction is the formation of a new electron‑pair bond. consider the chemical equation. n(g)+no(g)⟶nno(g) n(g)+no(g)⟶nno(g) write lewis formulas for the reactant and product species in the chemical equation. include nonbonding electrons. n(g)n(g) select draw rings more erase select draw rings more erase select draw rings more erase n no(g)
Answers: 1

You know the right answer?
Achemical equilibrium between gaseous reactants and products is shown. n2(g) + 3h2(g) ⇌ 2nh3(g) how...
Questions


Health, 25.03.2020 17:21

Mathematics, 25.03.2020 17:21

Mathematics, 25.03.2020 17:21

Mathematics, 25.03.2020 17:21

Mathematics, 25.03.2020 17:21

Biology, 25.03.2020 17:21




Physics, 25.03.2020 17:21





English, 25.03.2020 17:21

Mathematics, 25.03.2020 17:21


Arts, 25.03.2020 17:21

Mathematics, 25.03.2020 17:21