Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 20:30
What problem would a person have if the nucleic acid in one of his or her cells were damaged?
Answers: 2

Chemistry, 22.06.2019 07:00
The boiling point of propanoic acid is higher than that of 1-butanol because: propanoic acid has a higher molecular weight than 1-butanol. propanoic acid is more soluble in water than 1-butanol. propanoic acid is a better hydrogen bond donor than 1-butanol. propanoic acid forms hydrogen bonded dimers and 1-butanol does not. 1-butanol forms hydrogen bonded dimers and propanoic acid does not.
Answers: 2

Chemistry, 23.06.2019 19:00
What is the final temperature after 840 joules is absorbed by 10.0g of water at 25.0 c?
Answers: 1

Chemistry, 23.06.2019 21:30
Draw the structures of two different compounds that have the composition ch3no2. all three h atoms must remain bonded to the c atom and both o atoms must remain bonded to the n atom.. draw the molecules by placing atoms on the grid and connecting them with bonds. include all hydrogen atoms and nonbonding electrons.
Answers: 2
You know the right answer?
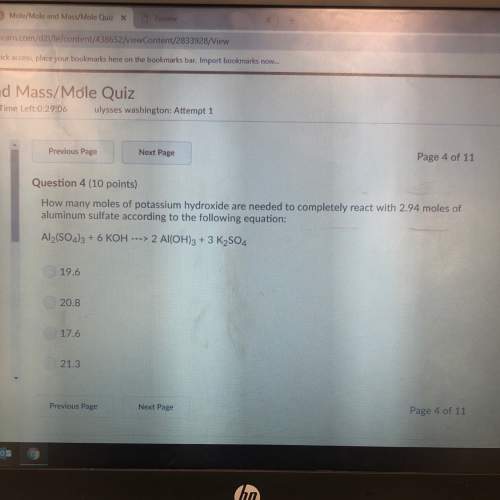
How many moles of potassium hydroxide are needed to completely react with 2.94 moles of aluminum sul...
Questions

Mathematics, 01.04.2021 06:50



English, 01.04.2021 06:50



Social Studies, 01.04.2021 06:50

Mathematics, 01.04.2021 06:50

Mathematics, 01.04.2021 06:50

Biology, 01.04.2021 06:50


English, 01.04.2021 06:50

Mathematics, 01.04.2021 06:50

History, 01.04.2021 06:50


Mathematics, 01.04.2021 06:50

Mathematics, 01.04.2021 06:50

Health, 01.04.2021 06:50

Spanish, 01.04.2021 06:50