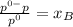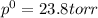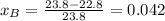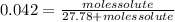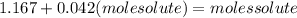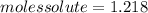Chemistry, 23.06.2019 09:00 blossie94681
The vapor pressure of water at 25.0°c is 23.8 torr. determine the mass of glucose (molar mass = 180 g/mol) needed to add to 500.0 g of water to change the vapor pressure to 22.8 torr.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 21:00
Write the chemical symbols for three different atoms or atomic cations with 27 electrons. asap!
Answers: 2

Chemistry, 22.06.2019 03:50
Consider the reaction: n2(g) + o2(g) ? 2no(g) kc = 0.10 at 2000oc starting with initial concentrations of 0.040 mol/l of n2 and 0.040 mol/l of o2, calculate the equilibrium concentration of no in mol/l how would this be done?
Answers: 3

Chemistry, 22.06.2019 06:30
Identify the missing numbers below to show the result of multiplying the numbers (1.6 × 10-19)(5.0 × 106) = c × 10d
Answers: 1

Chemistry, 22.06.2019 07:30
Compare and contrast the bohr model and the electron cloud models of the atom.
Answers: 1
You know the right answer?
The vapor pressure of water at 25.0°c is 23.8 torr. determine the mass of glucose (molar mass = 180...
Questions




Mathematics, 24.03.2021 19:00

Mathematics, 24.03.2021 19:00

Biology, 24.03.2021 19:00

Chemistry, 24.03.2021 19:00



History, 24.03.2021 19:00



Mathematics, 24.03.2021 19:00

Mathematics, 24.03.2021 19:00




Mathematics, 24.03.2021 19:00

Mathematics, 24.03.2021 19:00

Mathematics, 24.03.2021 19:00