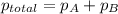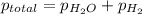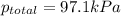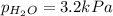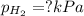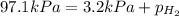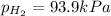Chemistry, 23.06.2019 10:30 fatheadd2007
When a chemist collects hydrogen gas over water, she ends up with a mixture of hydrogen and water vapor in her collecting bottle if the pressure in the collecting bottle is 97.1 kilopascals and the vapor pressure of the water is 3 2 kilopascals, what is the partial pressure of the hydrogen?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 13:40
Enough of a monoprotic weak acid is dissolved in water to produce a 0.0172 m solution. if the ph of the resulting solution is 2.39 at 20 °c, determine the pka for the acid.
Answers: 1


Chemistry, 22.06.2019 14:00
650.j is the same amount of energy as? 2720cal1550cal650.cal2.72cal
Answers: 2

Chemistry, 22.06.2019 19:50
When the mercury level in a barometer decreases that atmospheric pressure has
Answers: 3
You know the right answer?
When a chemist collects hydrogen gas over water, she ends up with a mixture of hydrogen and water va...
Questions


Social Studies, 13.02.2020 04:52







Mathematics, 13.02.2020 04:53



Mathematics, 13.02.2020 04:53

Mathematics, 13.02.2020 04:53

Mathematics, 13.02.2020 04:53



History, 13.02.2020 04:53

Mathematics, 13.02.2020 04:53

Mathematics, 13.02.2020 04:53