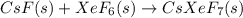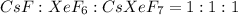Chemistry, 23.06.2019 14:00 kcutler8603
One of the few xenon compounds that form is cesium xenon heptafluoride (csxef7). how many moles of csxef7 can be produced from the reaction of 14.0 mol cesium fluoride with 14.0 mol xenon hexafluoride? csf(s) + xef6(s) csxef7(s)

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:30
You are to give ampicillin with a recommended dose of 25mg/kg to a child with a mass of 29kg. if stock on hand is 250mg/capsule how many capsules should be given?
Answers: 1

Chemistry, 22.06.2019 08:30
Identify one disadvantage to each of the following models of electron configuration: -dot structures -arrow and line diagrams -written electron configurations type in your answer below. (answer) -dot structures do not show the distribution of electrons in orbitals and take up a lot of space. -arrow and line diagrams take up a lot of space and make it difficult to count electrons. -written configurations make it easy to lose count of electrons and do not show the distribution of electrons in orbitals.
Answers: 3

Chemistry, 22.06.2019 13:50
How does the motion of particles in a gas change as the gas cools
Answers: 2

Chemistry, 22.06.2019 18:40
What is the binding energy of a nucleus that has a mass defect of 5.81*10-^29 kg a 5.23*10-^12 j b 3.15* 10^12 j c 1.57*10-3 j d 9.44*10^20 j
Answers: 1
You know the right answer?
One of the few xenon compounds that form is cesium xenon heptafluoride (csxef7). how many moles of c...
Questions

Geography, 06.10.2019 00:30



Arts, 06.10.2019 00:30



Computers and Technology, 06.10.2019 00:30

English, 06.10.2019 00:30




History, 06.10.2019 00:30

Mathematics, 06.10.2019 00:30



Computers and Technology, 06.10.2019 00:30




Chemistry, 06.10.2019 00:30

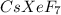 produced from the reaction is, 14 moles
produced from the reaction is, 14 moles