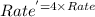Areaction in which a, b, and c react to form products is zero order in a, one-half order in b, and second order in c. by what factor does the reaction rate change if the concentration of a is doubled? 1 by what factor does the reaction rate change if the concentration of b is doubled? 1.4 by what factor does the reaction rate change if the concentration of c is doubled? 4

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 13:30
You 4. you have been swimming in your neighbor’s pool for an hour. the relative humidity of the air is 30 percent. will you feel warm or cool when you step out of the pool? explain your answer.
Answers: 1

Chemistry, 21.06.2019 16:00
Nickel crystallizes in the face-centered cubic (fcc) lattice. the density of the metal is 8902 kg/m3. calculate the radius of a nickel atom.
Answers: 1

Chemistry, 22.06.2019 15:00
Answer explain why it is not possible to deduce a complete order of reactivity.
Answers: 3

Chemistry, 22.06.2019 17:30
Air can be considered a mixture. which statement does not explain why?
Answers: 1
You know the right answer?
Areaction in which a, b, and c react to form products is zero order in a, one-half order in b, and s...
Questions

Biology, 30.10.2019 13:31



Mathematics, 30.10.2019 13:31

Social Studies, 30.10.2019 13:31



Physics, 30.10.2019 13:31

Mathematics, 30.10.2019 13:31



Mathematics, 30.10.2019 13:31

English, 30.10.2019 13:31

Mathematics, 30.10.2019 13:31

Mathematics, 30.10.2019 13:31


Chemistry, 30.10.2019 13:31


Mathematics, 30.10.2019 13:31

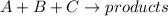
![Rate=k[A]^0[B]^\frac{1}{2}[C]^2](/tpl/images/0009/3165/73aa6.png)
![Rate^'=k[2A]^0[B]^\frac{1}{2}[C]^2](/tpl/images/0009/3165/fb0e0.png)
![Rate^'=k[2]^0[A]^0[B]^\frac{1}{2}[C]^2](/tpl/images/0009/3165/cb8ed.png)
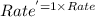
![Rate^'=k[A]^0[2B]^\frac{1}{2}[C]^2](/tpl/images/0009/3165/4f92e.png)
![Rate^'=k[A]^0[2]^\frac{1}{2}[B]^\frac{1}{2}[C]^2](/tpl/images/0009/3165/04cd0.png)
![Rate^'=[2]^\frac{1}{2}Rate](/tpl/images/0009/3165/3dc61.png)
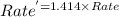
![Rate^'=k[A]^0[B]^\frac{1}{2}[2C]^2](/tpl/images/0009/3165/134f8.png)
![Rate^'=k[A]^0[B]^\frac{1}{2}[2]^2[C]^2](/tpl/images/0009/3165/77ebd.png)
![Rate^'=[2]^2Rate](/tpl/images/0009/3165/54b22.png)