
Chemistry, 30.11.2019 08:31 SuperWoman9172
Carbon disulfide burns in oxygen to yield car- bon dioxide and sulfur dioxide according to the chemical equation cs2(l) 3 o2(g) −→ co2(g) 2 so2(g). if 0.91 mol of cs2 is combined with 1.52 mol of o2, identify the limiting reactant.

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 06:30
Type the correct answer in the box. spell all words correctly.what is the correct term for living the most sustainable life you can within your current circumstances? when your are being as sustainable as you can within your current lifestyle, you are said to be sustainability.
Answers: 3

Chemistry, 22.06.2019 10:30
What determines the average kinetic energy of the particles in a gas? a. the number of collisions b. the number of particles c. the size of the particles d. the temperature
Answers: 1

You know the right answer?
Carbon disulfide burns in oxygen to yield car- bon dioxide and sulfur dioxide according to the chemi...
Questions


Mathematics, 25.01.2022 02:30

Chemistry, 25.01.2022 02:30

History, 25.01.2022 02:30


Mathematics, 25.01.2022 02:30


History, 25.01.2022 02:30

Biology, 25.01.2022 02:30


Mathematics, 25.01.2022 02:30


Mathematics, 25.01.2022 02:30



Mathematics, 25.01.2022 02:30



Mathematics, 25.01.2022 02:30

Chemistry, 25.01.2022 02:30

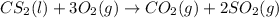
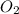 reacts with 1 mole of
reacts with 1 mole of 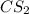
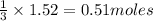 of
of 

