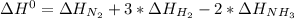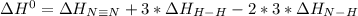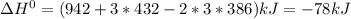Chemistry, 24.06.2019 21:30 gwoodbyrne
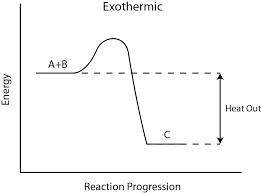
Given: n2 + 3h2 → 2nh3 bond bond energy (kj/mol) n≡n 942 h–h 432 n–h 386 use the bond energies to calculate the change in enthalpy for the reaction. the enthalpy change for the reaction is kilojoules.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:30
What does x represent in the formula for the compound xcl4?
Answers: 2

Chemistry, 22.06.2019 09:00
This chart lists four kinds of polymers and their sources. what can be known about all four polymers, despite their differences? they come from living things. they share ionic carbon bonds. they are at least 100 monomers long. they are made of repeating subunits.
Answers: 1


Chemistry, 23.06.2019 03:10
Which is true according to the law of conservation of energy
Answers: 1
You know the right answer?
Given: n2 + 3h2 → 2nh3 bond bond energy (kj/mol) n≡n 942 h–h 432 n–h 386 use the bond energies to c...
Questions














English, 30.10.2020 17:00

English, 30.10.2020 17:00





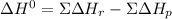 (2)
(2)