
In an experiment, two unknown compounds (one an alcohol and the other an ether) of equal molecular mass were dissolved in water. the result of the experiment is shown in the table. solubility comparison unknown compound solubility (g/100 ml water) unknown compound a--> solubility is 7.7 unknown compound b--> solubility is 6.8 which of the following correctly explains the identity of compound b and its solubility? a. it is an alcohol because the lack of hydrogen bonding between the oh^- make is less soluble b. it is an ether because it is unable to to form a hydrogen bond, so it is less soluble than water c. it is an ether because in the absence of a highly electronegative atom like o, f, or n, it lacks hydrogen bonding and thus makes it less soluble d. it is an alcohol because dispersion forces between the oh^- group and organic chain make it less soluble.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Which of these properties, used alone, would be least useful in identifying most minerals? a. color b. luster c. streak d. density
Answers: 2

Chemistry, 22.06.2019 06:00
Which of the following did jj thompson discover about atoms? a)an atom has an internal structure. b) atoms are tiny indivisible particles. c)electrons orbit the nucleus of an atom. d) the nucleus of an atom contains protons and neutrons.
Answers: 2


Chemistry, 22.06.2019 12:00
Which of the following units is not an official si unit? mole liter kilogram ampere
Answers: 1
You know the right answer?
In an experiment, two unknown compounds (one an alcohol and the other an ether) of equal molecular m...
Questions


Mathematics, 11.10.2020 02:01

Computers and Technology, 11.10.2020 02:01



Biology, 11.10.2020 02:01

Mathematics, 11.10.2020 02:01


Mathematics, 11.10.2020 02:01




Mathematics, 11.10.2020 02:01

Mathematics, 11.10.2020 02:01

History, 11.10.2020 02:01



Mathematics, 11.10.2020 02:01

History, 11.10.2020 02:01

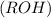 are more soluble in water as they can form hydrogen bonding with water whereas ethers
are more soluble in water as they can form hydrogen bonding with water whereas ethers 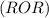 are less soluble as they do not form hydrogen bonds with water.
are less soluble as they do not form hydrogen bonds with water.

