Chemistry, 31.08.2019 03:30 carleygalloway103
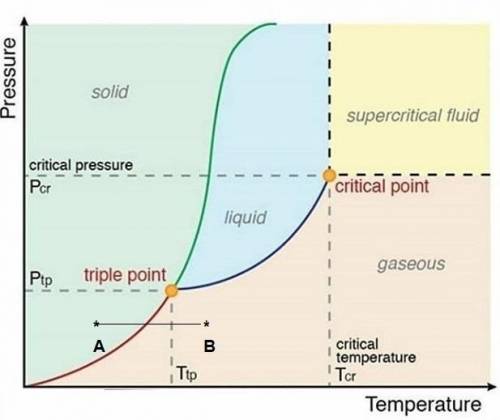
Asubstance has a triple point at -24.5 ∘c and 225 mmhg. what is most likely to happen to a solid sample of the substance as it is warmed from -35 ∘c to 0 ∘c at a pressure of 220 mmhg? a substance has a triple point at -24.5 and 225 . what is most likely to happen to a solid sample of the substance as it is warmed from -35 to 0 at a pressure of 220 ? the solid will melt into a liquid, the solid will sublime into a gas, or nothing (the solid will remain as a solid).

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:50
What is the temperature of one mole of helium gas at stp?
Answers: 3

Chemistry, 22.06.2019 05:00
Type the letter that represents the correct location for each particle type below.
Answers: 1


Chemistry, 22.06.2019 15:00
Phosphorous acid, h3po3(aq) , is a diprotic oxyacid that is an important compound in industry and agriculture. p k a1 p k a2 1.30 6.70 calculate the ph for each of the points in the titration of 50.0 ml of 1.5 m h3po3(aq) 1.5 m h 3 po 3 ( aq ) with 1.5 m koh(aq). 1.5 m koh ( aq ) .
Answers: 1
You know the right answer?
Asubstance has a triple point at -24.5 ∘c and 225 mmhg. what is most likely to happen to a solid sam...
Questions

History, 15.07.2019 10:20

History, 15.07.2019 10:20


Mathematics, 15.07.2019 10:20

Biology, 15.07.2019 10:20

Business, 15.07.2019 10:20

Social Studies, 15.07.2019 10:20

Social Studies, 15.07.2019 10:20



History, 15.07.2019 10:20





Chemistry, 15.07.2019 10:20

Social Studies, 15.07.2019 10:20

History, 15.07.2019 10:20

History, 15.07.2019 10:20

History, 15.07.2019 10:20