Chemistry, 25.06.2019 05:00 sarahaziz9526
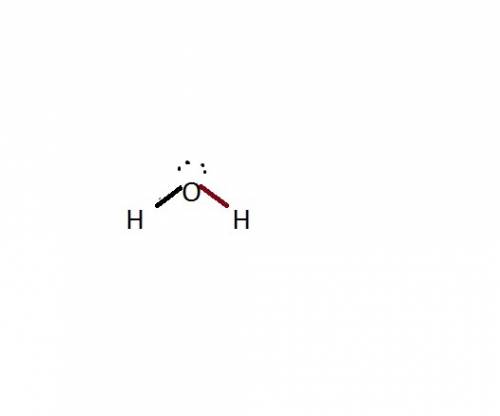
The common water molecule is polar because: it contains three polar covalent bonds. it contains one polar covalent bond. it contains no polar bonds, but is symmetrical. it has an asymmetrical shape.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 12:00
An atom's configuration based on its number of electrons ends at 3p4. another atom has seven more electrons. starting at 3p, what is the remaining configuration? 3p63d34s2 3p43d54s2 3p64s23d3 3p44s23d
Answers: 3

Chemistry, 22.06.2019 15:20
Which description best characterizes the motion of particles in a solid?
Answers: 2

Chemistry, 22.06.2019 20:50
What is the vapor pressure of a solution with a benzene to octane?
Answers: 2

Chemistry, 23.06.2019 09:00
How many moles of potassium hydroxide are needed to completely react with 2.94 moles of aluminum sulfate according to the following equation:
Answers: 2
You know the right answer?
The common water molecule is polar because: it contains three polar covalent bonds. it contains one...
Questions






Mathematics, 31.03.2020 18:22

Mathematics, 31.03.2020 18:22

Social Studies, 31.03.2020 18:22

History, 31.03.2020 18:22






Mathematics, 31.03.2020 18:22





English, 31.03.2020 18:23

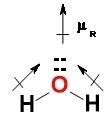
![:{\text{Number of electrons}} =\frac{1}{2}[V+N-C+A]](/tpl/images/0014/4487/08b6a.png)
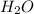
![{\text{Number of electrons}} =\frac{1}{2}[6+2-0+0]=4](/tpl/images/0014/4487/b0c43.png)
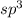 and the electronic geometry of the molecule will be tetrahedral.
and the electronic geometry of the molecule will be tetrahedral.