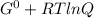Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 15:30
Why does earth rotate? because earth is formed from cold gases collapsing due to gravity because the matter in the nebula that formed earth was spinning because earth forms more than 99% of the mass of the solar system because the hydrogen atoms inside the nebula fused to form helium
Answers: 1

Chemistry, 22.06.2019 16:00
As changes in energy levels of electrons increase, the frequencies of atomic line spectra they emit
Answers: 2

Chemistry, 22.06.2019 22:10
What is the indicator of the number of ions in solution? the amount of conductivity the amount of precipitate the amount of solute added
Answers: 1

Chemistry, 22.06.2019 23:40
What energy conversion occurs when a sling shot is used to shoot a rock across the room? (2 points) question 2 options: 1) stored mechanical energy is converted to mechanical energy. 2) stored mechanical energy is converted to radiant energy. 3) gravitational energy is converted to radiant energy. 4) gravitational energy is converted to mechanical energy.
Answers: 1
You know the right answer?
What is δg for the formation of solid uranium hexafluoride from uranium and fluorine at 25∘c when th...
Questions



History, 12.12.2020 16:50



Social Studies, 12.12.2020 16:50

English, 12.12.2020 16:50


Mathematics, 12.12.2020 16:50


Chemistry, 12.12.2020 16:50


Mathematics, 12.12.2020 16:50

History, 12.12.2020 16:50


Mathematics, 12.12.2020 16:50

English, 12.12.2020 16:50

Geography, 12.12.2020 16:50


Spanish, 12.12.2020 16:50

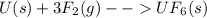
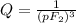
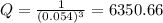
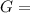 Δ
Δ