
Chemistry, 25.06.2019 05:30 sanociahnoel
If 3.8 moles of zinc metal react with 6.5 moles of silver nitrate, how many moles of silver metal can be formed, and how many moles of the excess reactant will be left over when the reaction is complete? unbalanced equation: zn + agno3 → zn(no3)2 + ag be sure to show all of your work.

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 00:00
Draw the skeletal structures of two different molecules that are each made of 5 carbon atoms and 12 hydrogen atoms.
Answers: 1

Chemistry, 22.06.2019 02:50
What is the overall order of reaction for rate = k[no]2[o2]
Answers: 3

Chemistry, 22.06.2019 13:00
Imagine that you push on a large rock. at what point does your effort change the rock’s mechanical energy?
Answers: 1
You know the right answer?
If 3.8 moles of zinc metal react with 6.5 moles of silver nitrate, how many moles of silver metal ca...
Questions

Mathematics, 18.11.2020 18:30

Mathematics, 18.11.2020 18:40

English, 18.11.2020 18:40


Biology, 18.11.2020 18:40


World Languages, 18.11.2020 18:40





Mathematics, 18.11.2020 18:40


World Languages, 18.11.2020 18:40


Chemistry, 18.11.2020 18:40



Mathematics, 18.11.2020 18:40

Mathematics, 18.11.2020 18:40

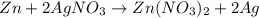
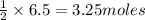 of zinc metal
of zinc metal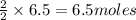 of silver metal.
of silver metal.

