Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 15:00
Which theory was contradicted by experiments with the photoelectric effect? light spreads out after it passes through a small opening. as soon as light strikes metal, electrons will be ejected. visible light, regardless of color, will cause the ejection of electrons when striking metal. the kinetic energy of ejected electrons depends on the frequency of light that strikes the metal.
Answers: 2

Chemistry, 23.06.2019 09:00
What properties would have caused early researchers to name hydrogen "inflammable air”
Answers: 3


Chemistry, 23.06.2019 13:00
If volume remains the same while the mass of a substance the density of the substance
Answers: 1
You know the right answer?
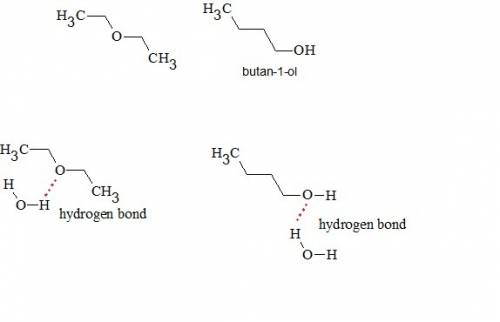
Diethyl ether and butan-1-ol are isomers, and they have similar solubilities in water. their boiling...
Questions



English, 26.05.2021 17:20



Mathematics, 26.05.2021 17:30

Mathematics, 26.05.2021 17:30

Mathematics, 26.05.2021 17:30






Mathematics, 26.05.2021 17:30


Mathematics, 26.05.2021 17:30

Mathematics, 26.05.2021 17:30


Mathematics, 26.05.2021 17:30

Mathematics, 26.05.2021 17:30