
Chemistry, 25.06.2019 13:30 cloeybrown
Which of the following has the greatest electronegativity difference between the bonded atoms? a. a strong acid made of hydrogen and a halogen, such as hcl b. carbon bonded to a group 6 a (16) nonmetal chalcogen, such as in co c. a diatomic gas, such as nitrogen (n2).d. a group 1 alkali metal bonded to iodide, such as nai.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:40
You may expect bonds between two atoms which each have n covalent lonic metallic hydrogen
Answers: 2

Chemistry, 21.06.2019 22:00
Afamily is one another name for a group on the table of elements.
Answers: 1


Chemistry, 23.06.2019 00:30
Which of the following best describes technology a. something created for only scientists to use b.the method of thinking that scientists use. c.the application of engineering to create useful products. c. a scientific idea
Answers: 1
You know the right answer?
Which of the following has the greatest electronegativity difference between the bonded atoms? a. a...
Questions

Social Studies, 27.06.2020 21:01

History, 27.06.2020 21:01














Mathematics, 27.06.2020 21:01




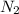 = Electronegativity value of nitrogen - electronegativity value of nitrogen
= Electronegativity value of nitrogen - electronegativity value of nitrogen

