Chemistry, 25.06.2019 19:00 blakestuhan
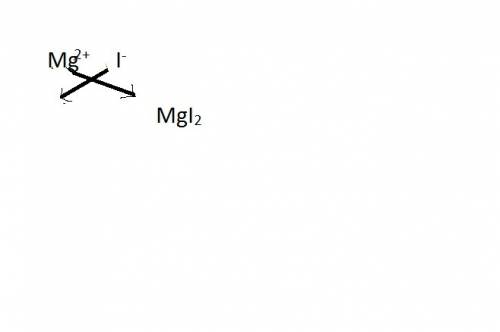
Select the properly written and balanced equation for the following chemical reaction: sodium metal and an aqueous solution of magnesium iodide react to produce an aqueous solution of sodium iodide and magnesium metal. na(s) + mgi (aq) -> nai (aq) + mg(s) na(s) + mgi2 (aq) -> nai2 (aq) + mg (s) 2na (s) + mgi2 (aq) -> 2nai (aq) + mg (s) 2na (s) + 4mgi (aq) -> 2nai2 (aq) + 4mg(s)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 12:30
Which element has the lowest electronegativity? calcium(ca) gallium(ga) selenium(se) bromine(br)
Answers: 1

Chemistry, 22.06.2019 20:00
For the reaction c6h14(g) & longrightarrow; c6h6(g) + 4h2(g), δp(h2)/δt was found to be 2.5 x 10-2 atm/s, where δp(h2) is the change in pressure of hydrogen. determine δp(c6h14)/δt for this reaction at the same time.
Answers: 2

Chemistry, 23.06.2019 04:20
The equation below shows a chemical reaction. a + b + heat —> c + d according to the law of conservation of energy, which statement is true? a. the reactants absorb heat because they have less energy than the products. b. the products release heat because they have more energy than the reactants. c. the reactants generate heat because they have more energy than the products. d. the products require heat to form because they have less energy than the reactants.
Answers: 1

Chemistry, 23.06.2019 05:30
Idont understand it 1.what is the boiling point of a solution of 675 grams of ethylene glycol (c2h6o2) in 2.50 liters of water?
Answers: 2
You know the right answer?
Select the properly written and balanced equation for the following chemical reaction: sodium metal...
Questions

Mathematics, 26.01.2021 20:50

Mathematics, 26.01.2021 20:50

History, 26.01.2021 20:50

Mathematics, 26.01.2021 20:50


History, 26.01.2021 20:50

Mathematics, 26.01.2021 20:50



Computers and Technology, 26.01.2021 20:50


Biology, 26.01.2021 20:50

English, 26.01.2021 20:50

Chemistry, 26.01.2021 20:50


Advanced Placement (AP), 26.01.2021 20:50




Mathematics, 26.01.2021 20:50

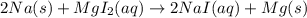
 .
.