
Chemistry, 25.06.2019 21:30 shyanne5276
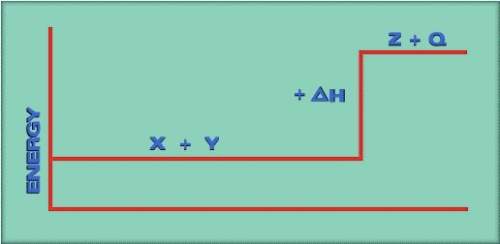
If we reverse the reaction to read z + q → x + y, the +δh would be a -δh. does this indicate that heat is given off or absorbed in the reverse reaction? -given off -absorbed

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 18:40
What is one real world example of a colligative property?
Answers: 2

Chemistry, 22.06.2019 19:20
Consider hydrogen in an excited state n = 5n=5 that emits photons to reach the ground state. there are various possible transitions other than straight to the ground state that can occur; for example, it can drop to the n = 3n=3 state followed by the n = 3n=3 to the ground state transition. which of the possible transitions will result in the emission of a photon in the visible region?
Answers: 3

Chemistry, 22.06.2019 20:00
In vapor-liquid equilibrium in a binary mixture, both components are generally present in both phases. how many degrees of freedom are there for such a system? the reaction between nitrogen and hydrogen to form ammonia occurs in the gas phase. how many degrees of freedom are there for this system? steam and coal react at high temperatures to form hydrogen, carbon monoxide, carbon dioxide, and methane. the following reactions have been suggested as being involved in the chemical transformation:
Answers: 3

Chemistry, 22.06.2019 21:30
What is another way to determine mass times acceleration?
Answers: 1
You know the right answer?
If we reverse the reaction to read z + q → x + y, the +δh would be a -δh. does this indicate that he...
Questions

English, 26.01.2021 01:00

Chemistry, 26.01.2021 01:00


History, 26.01.2021 01:00



English, 26.01.2021 01:00

Mathematics, 26.01.2021 01:00

Social Studies, 26.01.2021 01:00


Biology, 26.01.2021 01:00




Arts, 26.01.2021 01:00

Mathematics, 26.01.2021 01:00




English, 26.01.2021 01:00



