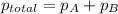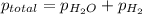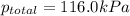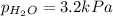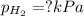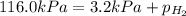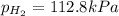Chemistry, 25.06.2019 23:00 allstar976
Asample of hydrogen gas is collected over water at 25°c. the total pressure of the hydrogen and water vapor is 116.0 kpa. the partial pressure of water vapor at this temperature is 3.2 kpa. what is the pressure of the hydrogen gas alone?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:50
Choose all that apply. when creating a graph, you should: determine the x- and y- variables label the scale on the x- and y- axes plot the data points draw a line of best fit to represent the data trend
Answers: 1

Chemistry, 22.06.2019 19:10
Astudent completes a titration by adding 12.0 milliliters of naoh(aq) of unknown concentration to 16.0 milliliters of 0.15 m hcl(aq). what is the molar concentration of the naoh(aq)? 1)5.0 m 2)0.20 m 3)0.11 m 4)1.1 m
Answers: 1

Chemistry, 23.06.2019 03:30
27 drag each label to the correct location on the image. a particular exosolar system has five planets in total: a, b, c, d, and e. the table lists the orbital periods of these planets in days. planet orbital period (days) a 600 b 80 c 1,000 d 500 e 100 move each planet to its orbit in the system.
Answers: 3

Chemistry, 23.06.2019 03:30
The molar mass of iron(fe) is 55.8 g/mol. what is the mass in grams of 2.25 moles of iron?
Answers: 1
You know the right answer?
Asample of hydrogen gas is collected over water at 25°c. the total pressure of the hydrogen and wate...
Questions

Spanish, 14.11.2019 15:31

Physics, 14.11.2019 15:31

Mathematics, 14.11.2019 15:31


Mathematics, 14.11.2019 15:31

Mathematics, 14.11.2019 15:31

Computers and Technology, 14.11.2019 15:31

Mathematics, 14.11.2019 15:31

Chemistry, 14.11.2019 15:31



Computers and Technology, 14.11.2019 15:31



Arts, 14.11.2019 15:31

Mathematics, 14.11.2019 15:31


English, 14.11.2019 15:31

Mathematics, 14.11.2019 15:31

Social Studies, 14.11.2019 15:31