
What is the theoretical yield of copper produced by the reaction of: 0.5 g al reacted with 3.5 g cucl2 x h2o? (cucl2 x h2o is a dihydrate when calculating the molecular weight) hint.) first find the limiting reactant, then the molecular weights of copper by the aluminum and the (copper chloride times water).

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 11:00
Freezing and boiling are endothermic processes. this means that these processes absorb energy from their surroundings in order to occur. use this information and the data you collected in the phase change gizmo to describe what happens to the temperature of water when you boil it, then explain why this result occurs.
Answers: 1

Chemistry, 22.06.2019 16:00
How does blood clotting prevent the entry of pathogens through cuts and wounds? answer asap,, this is due tomorrow. will mark as brainliest or whatever you call it : )
Answers: 2

Chemistry, 22.06.2019 17:00
Which statement is true about a catalyst? a: a catalyst decreases the rate of the reaction. b. a catalyst is consumed during a chemical reaction. c. a catalyst lowers the activation energy of a reaction. d. a catalyst increases the reactant concentration during a reaction.
Answers: 1

You know the right answer?
What is the theoretical yield of copper produced by the reaction of: 0.5 g al reacted with 3.5 g cu...
Questions



Mathematics, 22.09.2020 20:01

Mathematics, 22.09.2020 20:01

History, 22.09.2020 20:01



Physics, 22.09.2020 20:01

Mathematics, 22.09.2020 20:01

History, 22.09.2020 20:01

Biology, 22.09.2020 20:01



Mathematics, 22.09.2020 20:01





Social Studies, 22.09.2020 20:01

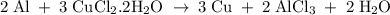
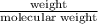
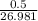
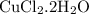 in reaction =
in reaction = 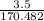
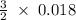
 170.482 grams of
170.482 grams of 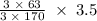 grams of Cu
grams of Cu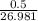 = 0.018 moles of Al.
= 0.018 moles of Al.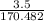 = 0.020 moles of CuCl₂.2H₂O.
= 0.020 moles of CuCl₂.2H₂O.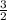 ×0.018 = 0.027.
×0.018 = 0.027.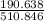 ×3.5 = 1.306g of Cu will produce.
×3.5 = 1.306g of Cu will produce.

