

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 13:00
What type of reaction is represented by the following example? 2co2 (g) + 4h2o (l) + 1452 kj 2ch3oh (l) (g) + 3o2 (g) exothermic endothermic
Answers: 1

Chemistry, 22.06.2019 09:30
What are scientists who study fossils called? ( a ) astronomers. ( b ) biologists. ( c ) geologists. ( d ) paleontologists.
Answers: 2

Chemistry, 22.06.2019 13:10
Which electron configuration represents the electrons in an atom of sodium in the ground state at stp
Answers: 1

Chemistry, 23.06.2019 04:00
Calculate the mass of 0.750 mol of the following substance. na3po4. , i'm not quite sure on how to set up the problem to solve! : (
Answers: 1
You know the right answer?
Refer to the balanced equation. ( quick) 8h2s+8cl2→s8+16hcl how many l of chlorine gas, at stp, wer...
Questions

History, 26.04.2021 23:10

Chemistry, 26.04.2021 23:10

Computers and Technology, 26.04.2021 23:10

Mathematics, 26.04.2021 23:10





Mathematics, 26.04.2021 23:10



Mathematics, 26.04.2021 23:10

Social Studies, 26.04.2021 23:10

English, 26.04.2021 23:10



English, 26.04.2021 23:10


Mathematics, 26.04.2021 23:10

Computers and Technology, 26.04.2021 23:10

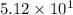 L
L 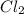
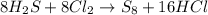
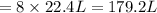 of chlorine gas.
of chlorine gas.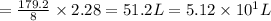 of chlorine gas.
of chlorine gas.

