Chemistry, 26.06.2019 09:00 jasonoliva13
Achemist wants to extract copper metal from copper chloride solution. the chemist places 0.25 grams of aluminum foil in a solution of 0.40 grams of copper (ii) chloride. a single replacement reaction takes place. what are the likely observations when the reaction stops? about 0.90 grams of copper (ii) is formed, and some aluminum is left in the reaction mixture. about 0.20 grams of copper (ii) is formed, and some aluminum is left in the reaction mixture. about 0.90 grams of copper (ii) is formed, and some copper chloride is left in the reaction mixture. about 0.20 grams of copper (ii) is formed, and some copper chloride is left in the reaction mixture.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 15:30
Which substance absorbs 58.16 kj of energy when 3.11 mol vaporizes? a)ch4 b)h2s c)co2 d)nacl
Answers: 2

Chemistry, 21.06.2019 18:40
Determine the energy released per kilogram of fuel used. given mev per reaction, calculate energy in joules per kilogram of reactants. consider 1 mole of tritium plus 1 mole of deuterium to be a mole of “reactions” (total molar mass = 5 grams).
Answers: 1

Chemistry, 22.06.2019 23:30
Match each statement with the state of matter it describes
Answers: 3

You know the right answer?
Achemist wants to extract copper metal from copper chloride solution. the chemist places 0.25 grams...
Questions










English, 20.03.2020 10:09



Chemistry, 20.03.2020 10:09



Mathematics, 20.03.2020 10:09



Chemistry, 20.03.2020 10:09

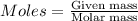
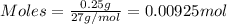
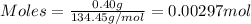
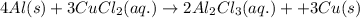
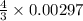 = 0.00396 moles of Aluminium.
= 0.00396 moles of Aluminium.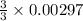 = 0.00297 moles of copper metal.
= 0.00297 moles of copper metal.