
Chemistry, 26.06.2019 11:30 tiffanydowell13
Iron(iii) oxide is formed when iron combines with oxygen in the air. how many moles of fe2o3 are formed when 55.8 g or fe reacts completely with oxygen? 4fe(s)+3o 2(g) --> 2fe2o3(s) a. 0.25 mol b. 0. 50 mol c. o. 75 mol d. 1.00 mol

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 17:00
An aqueous solution of hydroiodic acid is standardized by titration with a 0.186 m solution of calcium hydroxide. if 26.5 ml of base are required to neutralize 20.3 ml of the acid, what is the molarity of the hydroiodic acid solution? m hydroiodic acid
Answers: 1


Chemistry, 22.06.2019 00:00
1) these are barrel shaped microtubules in most animal cells, that organize the spindles during cell division
Answers: 1

Chemistry, 22.06.2019 23:00
What is the solubility-product constant of barium sulfate, baso4, if a saturated solution is 1.03 ´ 10-5 m?
Answers: 3
You know the right answer?
Iron(iii) oxide is formed when iron combines with oxygen in the air. how many moles of fe2o3 are for...
Questions




Mathematics, 11.11.2020 01:00

Mathematics, 11.11.2020 01:00

Biology, 11.11.2020 01:00




Social Studies, 11.11.2020 01:00

Mathematics, 11.11.2020 01:00

English, 11.11.2020 01:00



Mathematics, 11.11.2020 01:00

Mathematics, 11.11.2020 01:00

History, 11.11.2020 01:00

English, 11.11.2020 01:00


Mathematics, 11.11.2020 01:00

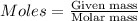
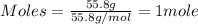
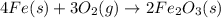
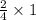 = 0.5 mole of Iron (III) oxide.
= 0.5 mole of Iron (III) oxide.

