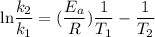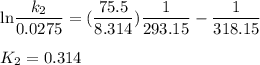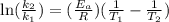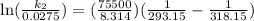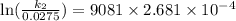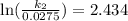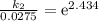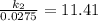Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 14:30
How many joules of heat are absorbed to raise the temperature of 650 grams of water from 5.00c to it's boiling point, 100c
Answers: 1

Chemistry, 22.06.2019 07:30
Given that 1 mi = 1760 yd, determine what conver- sion factor is appropriate to convert 1849 yd to miles; to convert 2.781 mi to yards.
Answers: 2

Chemistry, 22.06.2019 09:00
The nuclear fission process releases neutrons and question 27 options: alpha particles electrons energy beta particles
Answers: 1

Chemistry, 22.06.2019 10:40
If an area has high air pressure and low humidity, what type of weather will it most likely have? plz !
Answers: 1
You know the right answer?
Acertain first-order reaction has a rate constant of 2.75 10-2 s−1 at 20.°c. what is the value of k...
Questions

History, 19.09.2019 01:00

Social Studies, 19.09.2019 01:00

Mathematics, 19.09.2019 01:00

History, 19.09.2019 01:00

History, 19.09.2019 01:00




English, 19.09.2019 01:00



Mathematics, 19.09.2019 01:00

Mathematics, 19.09.2019 01:00



Mathematics, 19.09.2019 01:00

Engineering, 19.09.2019 01:00

History, 19.09.2019 01:00

Mathematics, 19.09.2019 01:00

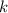 (rate constant) will be 0.314 s⁻¹ at 45°C if activation energy is 75.5 kJ/mol.
(rate constant) will be 0.314 s⁻¹ at 45°C if activation energy is 75.5 kJ/mol.