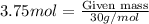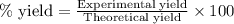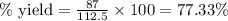Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 19:30
Complete the following reactions using word and balanced equations including states. dilute phosphoric acid is added with a calcium hydroxide solution.
Answers: 1

Chemistry, 22.06.2019 03:00
About 70 percent of the earth's surface is water-covered, and about 96.5 percent of all earth's water is salt water. identify the watery feature on earth that is made of freshwater rather than salt water. a) bay b) glacier c) ocean d) sea it is not incomplete this is the true question
Answers: 1

Chemistry, 22.06.2019 09:40
Consider this initial-rate data at a certain temperature for the reaction described by
Answers: 1

Chemistry, 22.06.2019 14:00
8.98 dm3 of hydrogen gas is collected at 38.8 °c. find the volume the gas will occupy at -39.9 °c if the pressure remains constant.
Answers: 3
You know the right answer?
The first step in the ostwald process for producing nitric acid is 4nh3(g) + 5o2(g) -> 4no(g) +...
Questions




Mathematics, 12.03.2020 00:42

Mathematics, 12.03.2020 00:42



Mathematics, 12.03.2020 00:42



Social Studies, 12.03.2020 00:42



Mathematics, 12.03.2020 00:42

English, 12.03.2020 00:43


History, 12.03.2020 00:43



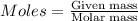
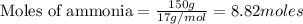
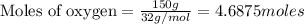
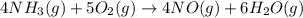
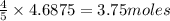 of ammonia
of ammonia