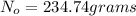Chemistry, 26.06.2019 15:00 imjustdumbsis
You are performing an experiment that uses 114ag. 113ag is radioactive, decays by beta-- emission and has a half-life of 21 minutes. it requires 4.2 hours to ship the material from the warehouse to your laboratory. how many grams should you order if the experiment requires that you have 0.0575 grams to begin the experiment?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:00
Will give brainliest it is a lab from k12 here is the linkfor each metal that participated in a chemical change, write the type of metal it is, based on your examination of the periodic table. type your answer here. (score for question 3: of 5 points) were there any metallic compounds that did not react with either the acid or the base? write the type of metal, based on your examination of the periodic table. type your answer here. (score for question 4: of 5 points) make a general statement about the reactivity of the metals in this experiment. type your answer here.
Answers: 2

Chemistry, 22.06.2019 04:00
You encounter a solution that is acidic and you decide to test it by adding a small amount of a strong acid. the ph lowers slightly but is approximately unchanged, and still remains acidic. what can you say about the solution? a. it is a buffer solution. b. it is not a buffer solution it is a strong acid solution. d. the solution has been neutralized. e. the solution has excess acid present
Answers: 1

Chemistry, 22.06.2019 05:00
Agas can holds 2.0 gal of gasoline. what is this quantity in cubic centimeters?
Answers: 2

Chemistry, 22.06.2019 09:30
Which formula can be used to calculate the molar mass of hydrogen peroxide
Answers: 1
You know the right answer?
You are performing an experiment that uses 114ag. 113ag is radioactive, decays by beta-- emission an...
Questions


Mathematics, 15.08.2020 01:01




Mathematics, 15.08.2020 01:01



Physics, 15.08.2020 01:01








Mathematics, 15.08.2020 01:01



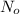
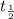 = 21 min
= 21 min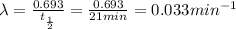
![\log[N]=\log[N_o]-\frac{\lambda t}{2.303}](/tpl/images/0019/8651/186bc.png)
![\log[0.0575 g]=\log[N_o]-\frac{0.033 min^{-1}\times 252 min}{2.303}](/tpl/images/0019/8651/dfaf0.png)