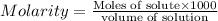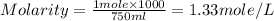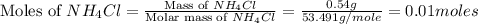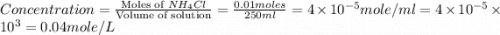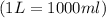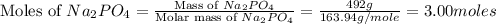Chemistry, 26.06.2019 15:30 genyjoannerubiera
1.distinguish between a 1m solution and a 1m solution. 2. calculate the molarity of 1.0 mol of kcl in 750 ml of solution. 3. what is the concentration (in m) of each of the following solutions? a. 0.54g of ammonium chloride in 250 ml of solution b. 492g of sodium phosphate in 500 ml of solution

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 21:40
During trial 2, what allowed you to determine that aluminum was the limiting reactant? check all that apply. all of the copper dissolved. all of the aluminum dissolved. the solution turned clear. the number of grams of copper(ii) chloride used in the reaction was greater than the number of grams of aluminum. the molar ratio of copper(ii) chloride to aluminum was greater than 3: 2, the equation’s molar ratio.
Answers: 2

Chemistry, 22.06.2019 05:50
According to coulomb's law, how would the electrical force between particles change if the product of their electrical charge increased?
Answers: 1

Chemistry, 22.06.2019 09:20
Give the orbital configuration of the phosphorus (p) atom.
Answers: 1

Chemistry, 22.06.2019 17:10
)benzene and toluene form nearly ideal solutions. consider an equimolar solution of benzene and toluene. at 20 °c the vapour pressures of pure benzene and toluene are 9.9 kpa and 2.9 kpa, respectively. the solution is boiled by reducing the external pressure below the vapour pressure. calculate (i) the pressure when boiling begins, (ii) the composition of each component in the vapour, and (iii) the vapour pressure when only a few drops of liquid remain. assume that the rate of vaporization is low enough for the temperature to remain constant at 20 °c.
Answers: 1
You know the right answer?
1.distinguish between a 1m solution and a 1m solution. 2. calculate the molarity of 1.0 mol of kcl...
Questions

Mathematics, 30.05.2021 06:00



Computers and Technology, 30.05.2021 06:00


Computers and Technology, 30.05.2021 06:00


Mathematics, 30.05.2021 06:00

Business, 30.05.2021 06:00


Social Studies, 30.05.2021 06:10


Chemistry, 30.05.2021 06:10

Mathematics, 30.05.2021 06:10

Geography, 30.05.2021 06:10



Mathematics, 30.05.2021 06:10

Mathematics, 30.05.2021 06:10

Mathematics, 30.05.2021 06:10