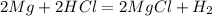Chemistry, 26.06.2019 17:00 catherinesquitieri
1. if you were to measure the mass of magnesium and hydrochloric acid before combining them in the test tube, how would that mass compare to the mass of reactants left in the test tube after the reaction? explain your answer and how it corresponds to the law of conservation of mass.

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 09:30
What are scientists who study fossils called? ( a ) astronomers. ( b ) biologists. ( c ) geologists. ( d ) paleontologists.
Answers: 2

Chemistry, 22.06.2019 10:40
If an area has high air pressure and low humidity, what type of weather will it most likely have? plz !
Answers: 1

Chemistry, 22.06.2019 11:50
If oil spills continue, all of the following should be expected except (2 points) death of aquatic life. polluted groundwater. decreased soil productivity. increased global temperatures.
Answers: 3
You know the right answer?
1. if you were to measure the mass of magnesium and hydrochloric acid before combining them in the t...
Questions




Biology, 26.03.2020 23:41




Mathematics, 26.03.2020 23:41

Mathematics, 26.03.2020 23:41


Physics, 26.03.2020 23:41

Mathematics, 26.03.2020 23:41


History, 26.03.2020 23:41

Mathematics, 26.03.2020 23:41



Mathematics, 26.03.2020 23:41