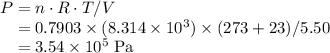Chemistry, 27.06.2019 00:30 abenjamin489
10.0 g of gaseous ammonia and 6.50 g of oxygen gas are introduced into a previously evacuated 5.50 l vessel. if the ammonia and oxygen then react to yield no gas and water vapor, what is the final gas pressure inside the vessel at 23? c

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 20:10
Suppose you mix one mole of sulfuric acid (h2so4) with 1 mole of sodium hydroxide(naoh). why does the ph of the solution remain below 7? ( explain so i can get better understanding! )
Answers: 2

Chemistry, 23.06.2019 00:00
Mercury turns to a vapor at 629.88 k. how much heat is lost when 75.0 g of mercury vapor at 650 k condenses to a liquid at 297 k?
Answers: 1


Chemistry, 23.06.2019 00:00
The empirical formula of a compound is ch2o and its mass is 120 amu/molecule, what is its formula?
Answers: 2
You know the right answer?
10.0 g of gaseous ammonia and 6.50 g of oxygen gas are introduced into a previously evacuated 5.50 l...
Questions




English, 21.06.2019 14:00

Biology, 21.06.2019 14:00








History, 21.06.2019 14:00

History, 21.06.2019 14:00

Mathematics, 21.06.2019 14:00


Mathematics, 21.06.2019 14:00

History, 21.06.2019 14:00

English, 21.06.2019 14:00