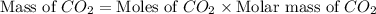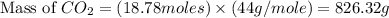Iron metal is obtained from the reaction of hematite [iron (iii) oxide, fe2o3] with carbon monoxide in a blast furnace. fe2o3 (s) + 3 co (g) > 2 fe (s) + 3 co2 (g) (a) calculate the number of grams of iron metal that can be obtained from 1.00 kg of hematite (assuming that you have enough co available for any reaction). feb) calculate the amount of co2 in grams that you you will get in this reaction, using the amount of hematite in (a). g co2

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 07:20
Which of these conditions most likely produces an unstable isotope?
Answers: 1

Chemistry, 22.06.2019 08:40
Which statement can best be concluded from the ideal gas law?
Answers: 2

Chemistry, 22.06.2019 15:30
The gulf stream is a warm water current that flows away from the equator to northern europe. witch of these does it cause. a. crashes of warm and cool water in the ocean b.colder climates near the equator c.large waves on the cost of europe d.warm climates in northern europe
Answers: 1

Chemistry, 22.06.2019 20:20
Nitric acid can be formed in two steps from the atmospheric gases nitrogen and oxygen, plus hydrogen prepared by reforming natural gas. in the first step, nitrogen and hydrogen react to form ammonia: (g) (g) (g) in the second step, ammonia and oxygen react to form nitric acid and water: (g) (g) (g) (g) calculate the net change in enthalpy for the formation of one mole of nitric acid from nitrogen, hydrogen and oxygen from these reactions. round your answer to the nearest .
Answers: 3
You know the right answer?
Iron metal is obtained from the reaction of hematite [iron (iii) oxide, fe2o3] with carbon monoxide...
Questions

History, 05.12.2019 20:31





Mathematics, 05.12.2019 20:31


English, 05.12.2019 20:31

Mathematics, 05.12.2019 20:31

Mathematics, 05.12.2019 20:31

English, 05.12.2019 20:31

Mathematics, 05.12.2019 20:31






English, 05.12.2019 20:31

Mathematics, 05.12.2019 20:31

Mathematics, 05.12.2019 20:31

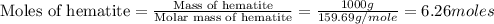
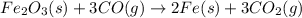
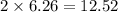 moles of iron
moles of iron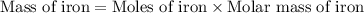
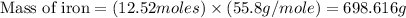
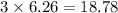 moles of carbon dioxide
moles of carbon dioxide