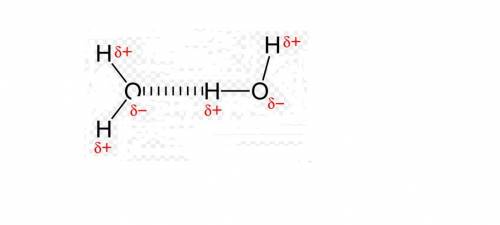
Which sentence best explains the high melting point, boiling point, and surface tension of water? a. the hydrogen atom on each water molecule is strongly attracted to the hydrogen atoms on nearby molecules. b. ions dissolved in the water cause nearby molecules to become temporarily polar. c. the water molecules are connected to each other with covalent bonds. d. water molecules that have lost electrons attract the water molecules that have gained electrons. e. the negative side of each water molecule is attracted to the positive sides of nearby molecules.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 08:30
Which of the following would have less momentum than a 52 kg cheetah running at 10 m/s?
Answers: 2

Chemistry, 22.06.2019 22:30
Is the idea of spontaneous generation supported by redi's experiment? justify your answer in 2-3 sentences?
Answers: 1

Chemistry, 23.06.2019 13:50
Use the periodic table and your knowledge of isotopes to complete these statements. when polonium-210 emits an alpha particle, the child isotope has an atomic mass of 1-131 undergoes beta-minus decay. the chemical symbol for the new element is fluorine-18 undergoes beta-plus decay. the child isotope has an atomic mass of done intro donne
Answers: 1

Chemistry, 23.06.2019 17:30
Consider the lewis structure for ch4 methane is this molecule polar or nonpolar
Answers: 1
You know the right answer?
Which sentence best explains the high melting point, boiling point, and surface tension of water? a...
Questions


Mathematics, 31.10.2019 22:31

History, 31.10.2019 22:31

Physics, 31.10.2019 22:31


Mathematics, 31.10.2019 22:31

History, 31.10.2019 22:31

Physics, 31.10.2019 22:31

Biology, 31.10.2019 22:31

English, 31.10.2019 22:31


Mathematics, 31.10.2019 22:31