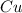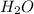Chemistry, 27.06.2019 02:30 avanelson01
An individual with an excellent sense of smell is handed a bottle of perfume. after smelling the perfume, he reports that the perfume contains a predominant smell of roses with a faint touch of vanilla. if the perfume is made from water, rose essence, and a splash of vanilla, it is most likely a. a mixture. b. a gas. c. an element. d. a compound.

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 19:00
Convert the temperature of dry ice, –77 ∞c, into degrees fahrenheit and kelvin.
Answers: 2

Chemistry, 23.06.2019 00:00
#7 how does the structure of amino acids allow them to form a polypeptide? each amino acid has an amino group and a carboxyl group. each amino acid has a hydrogen atom and a carboxyl group. each amino acid has a carboxyl group and an r group. each amino acid has an r group and a hydrogen atom.
Answers: 1

Chemistry, 23.06.2019 05:00
Which of the following describes qualitative data? a) recording the temperature of a solid as it is warmed. b) noting the color of a solution as it is heated. c) measuring the volume of an object by water displacement. d) taking the mass of an object using a balance.
Answers: 2
You know the right answer?
An individual with an excellent sense of smell is handed a bottle of perfume. after smelling the per...
Questions




Mathematics, 14.09.2021 04:40


Physics, 14.09.2021 04:40




Chemistry, 14.09.2021 04:40



History, 14.09.2021 04:50




English, 14.09.2021 04:50

History, 14.09.2021 04:50

Mathematics, 14.09.2021 04:50

English, 14.09.2021 04:50