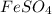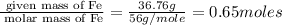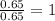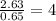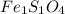Chemistry, 27.06.2019 03:00 ronaldhernandez598
What is the empirical formula? a compound is used to treat iron deficiency in people. it contains 36.76% iron, 21.11% sulfur, and 42.13% oxygen. the empirical formula is feso. reset next

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 17:00
What mass of carbon dioxide is produced from the complete combustion of 4.50×10−3 g of methane?
Answers: 2

Chemistry, 22.06.2019 03:10
The covalent compound acetylene, which is the fuel of the oxyacetylene torch used by welders, has the molecular formula c2h2. the covalent compound benzene, a commercial solvent, has the molecular formula c6h6 each of these covalent compounds contains carbon and hydrogen atoms in a one-to-one ratio. would it be correct to write the chemical formulas of each as ch? explain.
Answers: 1

Chemistry, 22.06.2019 10:00
Part 1: include important facts found through your research. part 2: include your visual display. include your summary of “the chemistry of water” from the national science foundation website. include your experiment. part 3: include responses to the reflection questions.
Answers: 1

Chemistry, 22.06.2019 13:00
The molality of calcium chloride (cacl2) in an aqueous solution is 2.46 m. what is mole fraction of the solute?
Answers: 3
You know the right answer?
What is the empirical formula? a compound is used to treat iron deficiency in people. it contains 3...
Questions


English, 24.06.2020 08:01

Mathematics, 24.06.2020 08:01

English, 24.06.2020 08:01

History, 24.06.2020 08:01

History, 24.06.2020 08:01

Mathematics, 24.06.2020 08:01

Mathematics, 24.06.2020 08:01




Mathematics, 24.06.2020 08:01






Mathematics, 24.06.2020 08:01