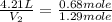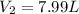Chemistry, 27.06.2019 03:00 paulesparsa6
If 27.1 g of ar(g) occupies a volume of 4.21 l, what volume will 1.29 moles of ne(g) occupy at the same temperature and pressure?

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 01:30
Agas is contained in a thick walled balloon when the pressure changes from 1.21 atm to 2.52 the volume changes from 3.75 l to 1.72 l and the temperature change from 293k to blank k
Answers: 3

Chemistry, 22.06.2019 12:10
If a molecule with a molecular formula of c13h18 is treated with an excess of h2 in the presence of finally divided pt metal under conditions required for maximum hydrogenation of the molecule to give a molecule with a formula c13h24, how many rings are in the molecule?
Answers: 3

Chemistry, 22.06.2019 14:30
Connect the whole numbers on the periodic table to indicate what they represent?
Answers: 3
You know the right answer?
If 27.1 g of ar(g) occupies a volume of 4.21 l, what volume will 1.29 moles of ne(g) occupy at the s...
Questions

Health, 02.09.2019 12:10




French, 02.09.2019 12:10

Social Studies, 02.09.2019 12:10

Mathematics, 02.09.2019 12:10

Chemistry, 02.09.2019 12:10

Social Studies, 02.09.2019 12:10


Mathematics, 02.09.2019 12:10


Biology, 02.09.2019 12:10

Chemistry, 02.09.2019 12:10

Mathematics, 02.09.2019 12:10

Biology, 02.09.2019 12:10


Social Studies, 02.09.2019 12:10

Mathematics, 02.09.2019 12:10

History, 02.09.2019 12:10

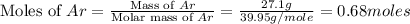
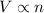
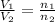
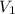 = volume of argon gas
= volume of argon gas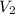 = volume of neon gas
= volume of neon gas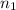 = number of moles of argon gas
= number of moles of argon gas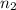 = number of moles of neon gas
= number of moles of neon gas