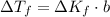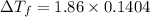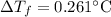Chemistry, 27.06.2019 05:30 tonhill6923
Can someone me ? 13. the boiling point of a solvent is elevated by 2.4 °c when the solute concentration is 3.1 m. what is kb? what is the freezing-point depression of a solution that contains 0.705 mol of a nonelectrolyte solute in 5.02 kg of water? (kf = 1.86 °c/m)

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 18:00
During which movies do spring tides new moon first quarter waxing gibbous waxing
Answers: 1

Chemistry, 21.06.2019 18:10
Using complete sentences, explain how to predict the products and balance the reaction between sulfuric acid and potassium hydroxide.
Answers: 1

Chemistry, 21.06.2019 22:10
How do forces between particles in gases compare to forces in the other states of matter? o a. the forces in gases are stronger than forces in solids but weaker than forces in liquids. o b. the forces in gases are weaker than forces in solids but stronger than forces in liquids. o c. the forces in gases are weaker than forces in solids and liquids. o d. the forces in gases are stronger than forces in solids and liquids. submit
Answers: 1

Chemistry, 22.06.2019 11:30
For each of the following compounds, decide whether the compound's solubility in aqueous solution changes with ph. if the solubility does change, pick the ph at which you'd expect the highest solubility. you'll find ksp data in the aleks data tab. compounds does solubility change with ph
Answers: 3
You know the right answer?
Can someone me ? 13. the boiling point of a solvent is elevated by 2.4 °c when the solute concent...
Questions

Mathematics, 28.03.2020 03:02


Spanish, 28.03.2020 03:02








Mathematics, 28.03.2020 03:17



English, 28.03.2020 03:18

Social Studies, 28.03.2020 03:18





 is
is