
Chemistry, 27.06.2019 06:00 brooke0713
Complete the paragraph to describe the characteristics of silicon tetrachloride molecule (sih4). the lewis structure and the table of electronegativities are given. the bond polarities of sih4 are the molecular shape is and the molecule is blank options 1: polar, nonpolar blank options 2: trigonal pyramidal, trigonal planar, tetrahedral, linear, bentblank options 3: polar, nonpolar
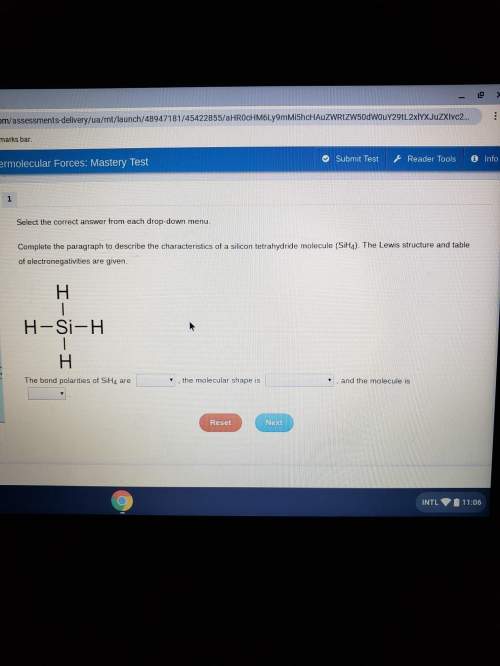

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 18:20
Which reason best explains why metals are malleable? a)because they have delocalized electrons b)because they have localized electrons c)because they have ionic bonds d)because they have rigid bonds
Answers: 2

Chemistry, 22.06.2019 18:30
Which of the following nuclei would be the least stable a 2 protons, 2 neutrons b 1 proton 1 neutron c 1 proton 3 neutrons d 1 proton 2 neutrons
Answers: 3

Chemistry, 22.06.2019 19:00
Structure of the atoms: discovery of the nucleus in 1909i need answering all of these questions
Answers: 3

Chemistry, 23.06.2019 00:20
4. propanol and isopropanol are isomers. this means that they have a) the same molecular formula but different chemical properties. b) different molecular formulas but the same chemical properties. c) the same molecular formula and the same chemical properties. d) the same molecular formula but represent different states of the compound
Answers: 3
You know the right answer?
Complete the paragraph to describe the characteristics of silicon tetrachloride molecule (sih4). the...
Questions



Mathematics, 01.12.2021 17:40

SAT, 01.12.2021 17:40

Geography, 01.12.2021 17:40

Health, 01.12.2021 17:40

Chemistry, 01.12.2021 17:40




Computers and Technology, 01.12.2021 17:40




English, 01.12.2021 17:40

Physics, 01.12.2021 17:40

Mathematics, 01.12.2021 17:40

Mathematics, 01.12.2021 17:40

Mathematics, 01.12.2021 17:40

English, 01.12.2021 17:40



