
Chemistry, 27.06.2019 08:30 quintinjerome
Hurry ! calculating - a sample it barium nitrate is placed into a jar containing water. the mass of the barium nitrate sample is 27g. assume the water is at 20°c and that the resulting barium nitrate solution is saturated. what mass of water is present in the jar?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 12:30
If anyone would be able to me out with these three questions it would be these are from the chem 2202 course.
Answers: 3

Chemistry, 22.06.2019 18:00
Heat is the total potential energy of a substance that can be transferred. true false
Answers: 1

Chemistry, 23.06.2019 04:00
Achemical reaction is done in the setup shown , resulting in a change of mass. what will happen if the same reaction is done in a sealed container that is placed on the electronic balance?
Answers: 2

You know the right answer?
Hurry ! calculating - a sample it barium nitrate is placed into a jar containing water. the mass of...
Questions

Mathematics, 02.06.2021 18:20


English, 02.06.2021 18:20


Mathematics, 02.06.2021 18:20

Mathematics, 02.06.2021 18:20



Geography, 02.06.2021 18:20

English, 02.06.2021 18:20




Mathematics, 02.06.2021 18:20

Chemistry, 02.06.2021 18:20




English, 02.06.2021 18:20

Mathematics, 02.06.2021 18:20

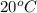 is 9.02 gram per 100 ml of water.
is 9.02 gram per 100 ml of water.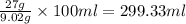 of water
of water



